Families of children with neuroblastoma await the Ministry of Health’s decision on the possibility of incorporating the high-cost drug Qarziba into the list of medicines distributed by the Unified Health System. The Recordati pharmaceutical laboratory submitted the medicine for evaluation by the National Commission for the Incorporation of Technologies in the Unified Health System (Conitec) on January 17th, and the analysis period is 180 days, extendable for another 90 days.
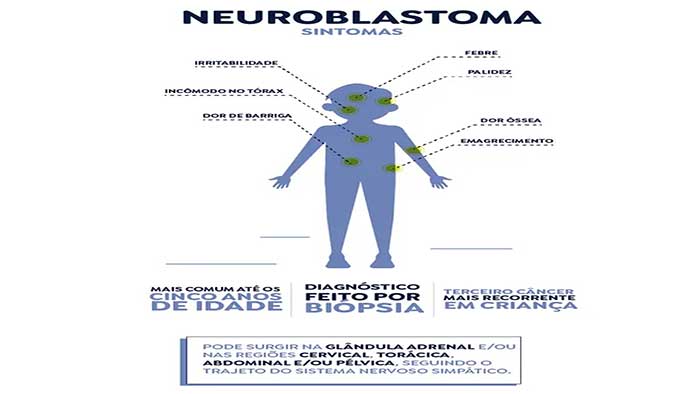
Neuroblastoma is the third most common type of childhood cancer, after leukemia and brain tumors. The medicine, which costs around R$2 million, is indicated for high-risk cases or recurrence and has already been used in more than a thousand patients in 18 countries. According to the manufacturer, it improves survival, increases the likelihood of cure and reduces the risk of the disease returning.
The Ministry of Health reported that the drug evaluation process involves the severity of the cases, the characteristics of the disease, the drug’s potential to transform patients’ quality of life and costs.