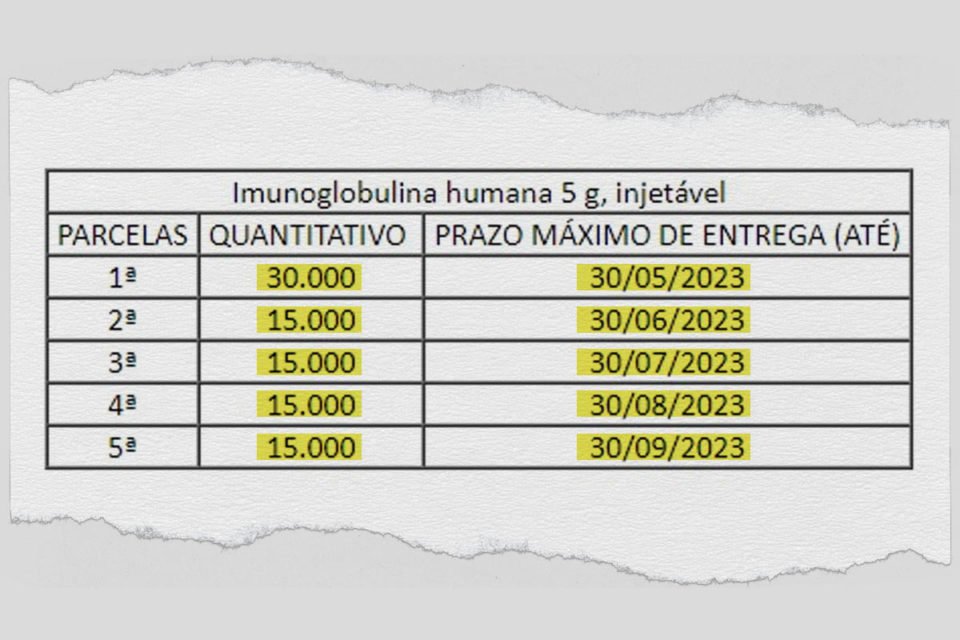
The Ministry of Health hired a company exempt from bidding to supply 90,000 vials of human immunoglobulin, for R$87 million, but has not yet received any units of the medicine. The contract was signed in April, with five installments expected to be delivered by September 30th.
“To date, there is no record of receipt of inputs, and, as a result, no payments have been made to the company,” stated the ministry.
The company in question is Farma Medical, which signs the contract as national representative of Prime Pharma LLC, from the United Arab Emirates. In a note, she guarantees that the first batches have been available since June 13th.
“The import of said product is the sole and exclusive responsibility of the Ministry of Health,” he said. According to Farma Medical, availability occurred 13 days after the exceptional import authorization issued by the National Health Surveillance Agency (Anvisa). Authorization was necessary because the medicine being offered by the company is not registered with the agency.
The company’s owner, Silvio de Azevedo Pereira Júnior, said that 30 thousand bottles were actually delivered to the ministry in June. The report requested access to supporting documents, but they were not made available.
Farma Medical has share capital of R$100 million and is present in six states.
In the pantry
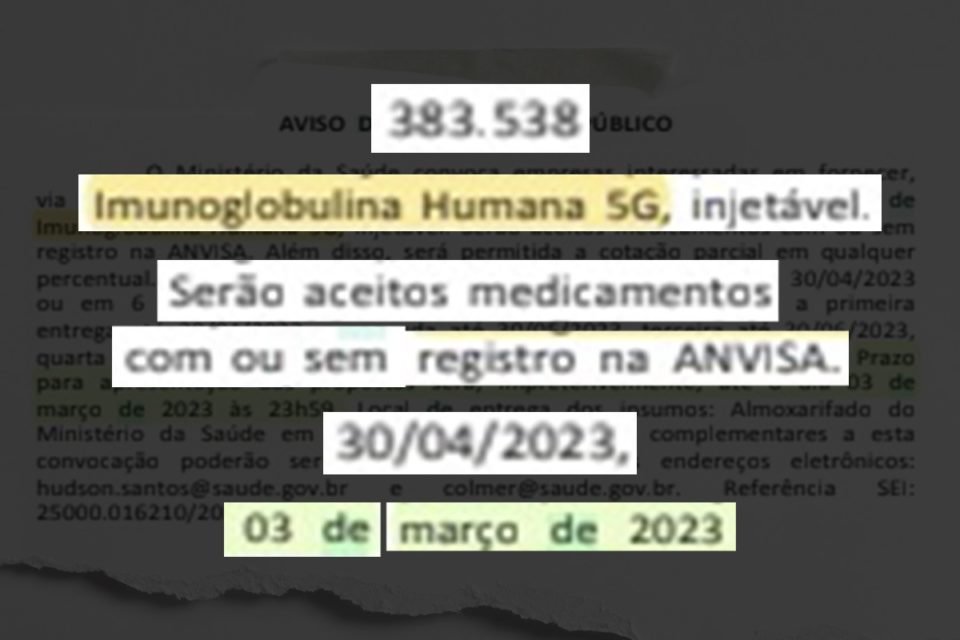
At the end of February, the ministry opened a process for the purchase of 383,500 vials of immunoglobulin with an exemption from bidding, indicating urgency. In total, 20 companies sent their proposals, and Prime Pharma’s was among the most advantageous.
When claiming urgency, the ministry stated that the first installment of medicine should be delivered in April, to avoid the risk of shortages. In the end, the first installment was only delivered in mid-June by the company Auramedi Farmacêutica, which won the majority of the purchase.
Immunoglobulin is a blood-derived medicine, that is, produced from blood, used to improve the immunity of patients affected by a series of diseases, such as Guillain-Barré syndrome. Its lack in the Unified Health System (SUS) puts the lives of people who depend on the medicine at risk.
Delay
After the contract was signed, on April 14, the ministry would need to ask Anvisa for authorization for the import on an exceptional basis. The ministry then requested the documentation from the Brazilian company and made the request on May 16th. The agency only authorized the import on the 29th of that month, which would make it impossible, in any case, for the first installment to be delivered within the deadline, which was the 30th.
According to documents from the ministry, only on June 20th, the Ministry’s Logistics Planning and Control Department sent a letter to the Pharmaceutical Assistance area informing Anvisa’s decision, 10 days before the second installment was due.
Silvio Júnior, from Farma Medical, argued that the department took longer to approve the documents sent by his company than the time dedicated to its competitor, Auramedi. “I don’t know why, but it took a lot longer to approve,” he said.

Breach of contract
On July 28, the ministry sent a notice of breach of contract claiming that the first installment had not yet been delivered. On August 22, the department sent another demand, pointing out delays in the first three installments.
Farma Medical admits delays in the contractual execution schedule, but emphasizes that this initially occurred “due to the ministry’s own procedures, which on the first delivery date stipulated in the contract did not even obtain exceptional authorization from Anvisa for imports”.
“This company did not fail to fulfill its obligations as a national representative, nor did the direct suppliers of the Ministry of Health”, he argued.
Nanjing
With only one employee registered at least until March and share capital of R$1.3 million, Auramedi, from Goiás, was awarded a contract worth R$285.8 million within the scope of this same bidding exemption process. The company claims to represent the Chinese Nanjing Pharmacare nationally.
As Metrópoles showed last Tuesday (26/9), Nanjing is also represented in Brazil by Panamerican Medical Supply, one of whose partners is Marcelo Pupkin Pitta, a businessman in the field who was arrested in Operation Vampiro in 2004 , and, again, in 2007.
The investigations revealed suspicion of fraud in tenders at the Ministry of Health, precisely in purchases of blood products, including immunoglobulin.
Panamerican even signed two contracts worth R$647.2 million with the ministry under the government of former president Jair Bolsonaro (PL), in 2021 and 2022, to supply immunoglobulin as a representative of Nanjing. At least R$597.5 million has already been paid, according to the Transparency Portal, 73.1% of which was disbursed last year, between March and September, and the rest this year, already during the Lula government.
Source: Metropolises
The Ministry of Health hired a company exempt from bidding to supply 90,000 vials of human immunoglobulin, for R$87 million, but has not yet received any units of the medicine. The contract was signed in April, with five installments expected to be delivered by September 30th.
“To date, there is no record of receipt of inputs, and, as a result, no payments have been made to the company,” stated the ministry.
The company in question is Farma Medical, which signs the contract as national representative of Prime Pharma LLC, from the United Arab Emirates. In a note, she guarantees that the first batches have been available since June 13th.
“The import of said product is the sole and exclusive responsibility of the Ministry of Health,” he said. According to Farma Medical, availability occurred 13 days after the exceptional import authorization issued by the National Health Surveillance Agency (Anvisa). Authorization was necessary because the medicine being offered by the company is not registered with the agency.
The company’s owner, Silvio de Azevedo Pereira Júnior, said that 30 thousand bottles were actually delivered to the ministry in June. The report requested access to supporting documents, but they were not made available.
Farma Medical has share capital of R$100 million and is present in six states.
In the pantry
At the end of February, the ministry opened a process for the purchase of 383,500 vials of immunoglobulin with an exemption from bidding, indicating urgency. In total, 20 companies sent their proposals, and Prime Pharma’s was among the most advantageous.
When claiming urgency, the ministry stated that the first installment of medicine should be delivered in April, to avoid the risk of shortages. In the end, the first installment was only delivered in mid-June by the company Auramedi Farmacêutica, which won the majority of the purchase.
Immunoglobulin is a blood-derived medicine, that is, produced from blood, used to improve the immunity of patients affected by a series of diseases, such as Guillain-Barré syndrome. Its lack in the Unified Health System (SUS) puts the lives of people who depend on the medicine at risk.
Delay
After the contract was signed, on April 14, the ministry would need to ask Anvisa for authorization for the import on an exceptional basis. The ministry then requested the documentation from the Brazilian company and made the request on May 16th. The agency only authorized the import on the 29th of that month, which would make it impossible, in any case, for the first installment to be delivered within the deadline, which was the 30th.
According to documents from the ministry, only on June 20th, the Ministry’s Logistics Planning and Control Department sent a letter to the Pharmaceutical Assistance area informing Anvisa’s decision, 10 days before the second installment was due.
Silvio Júnior, from Farma Medical, argued that the department took longer to approve the documents sent by his company than the time dedicated to its competitor, Auramedi. “I don’t know why, but it took a lot longer to approve,” he said.



Breach of contract
On July 28, the ministry sent a notice of breach of contract claiming that the first installment had not yet been delivered. On August 22, the department sent another demand, pointing out delays in the first three installments.
Farma Medical admits delays in the contractual execution schedule, but emphasizes that this initially occurred “due to the ministry’s own procedures, which on the first delivery date stipulated in the contract did not even obtain exceptional authorization from Anvisa for imports”.
“This company did not fail to fulfill its obligations as a national representative, nor did the direct suppliers of the Ministry of Health”, he argued.
Nanjing
With only one employee registered at least until March and share capital of R$1.3 million, Auramedi, from Goiás, was awarded a contract worth R$285.8 million within the scope of this same bidding exemption process. The company claims to represent the Chinese Nanjing Pharmacare nationally.
As Metrópoles showed last Tuesday (26/9), Nanjing is also represented in Brazil by Panamerican Medical Supply, one of whose partners is Marcelo Pupkin Pitta, a businessman in the field who was arrested in Operation Vampiro in 2004 , and, again, in 2007.
The investigations revealed suspicion of fraud in tenders at the Ministry of Health, precisely in purchases of blood products, including immunoglobulin.
Panamerican even signed two contracts worth R$647.2 million with the ministry under the government of former president Jair Bolsonaro (PL), in 2021 and 2022, to supply immunoglobulin as a representative of Nanjing. At least R$597.5 million has already been paid, according to the Transparency Portal, 73.1% of which was disbursed last year, between March and September, and the rest this year, already during the Lula government.
Source: Metropolises






